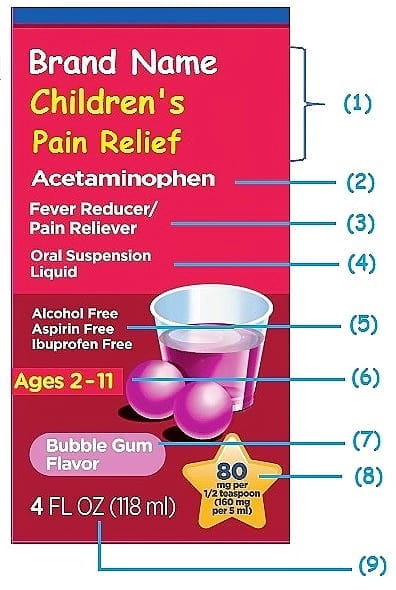
39 medication labels must include
FDA Issues New RX Label Requirements - The Recovery Village Drug and ... A prescription drug label must include certain information. FDA prescription labeling requirements must be clearly printed with: Pharmacy information Doctor information Instructions Physical description of the drug Federal caution statement Dates Pharmacy prescription number Number of pills Number of times the drug can be reordered Pharmaceutical Labeling: Requirements & Guidelines - CTM Labeling Systems To meet today's FDA regulations, labeling information on drugs must include the following in this order: - Product Name - Drug Facts Table - Active Ingredients - Purpose and Use - Warnings - Directions - Allergic Reactions - Inactive Ingredients
Health News | Latest Medical, Nutrition, Fitness News - ABC News - ABC News Oct 06, 2022 · Get the latest health news, diet & fitness information, medical research, health care trends and health issues that affect you and your family on ABCNews.com
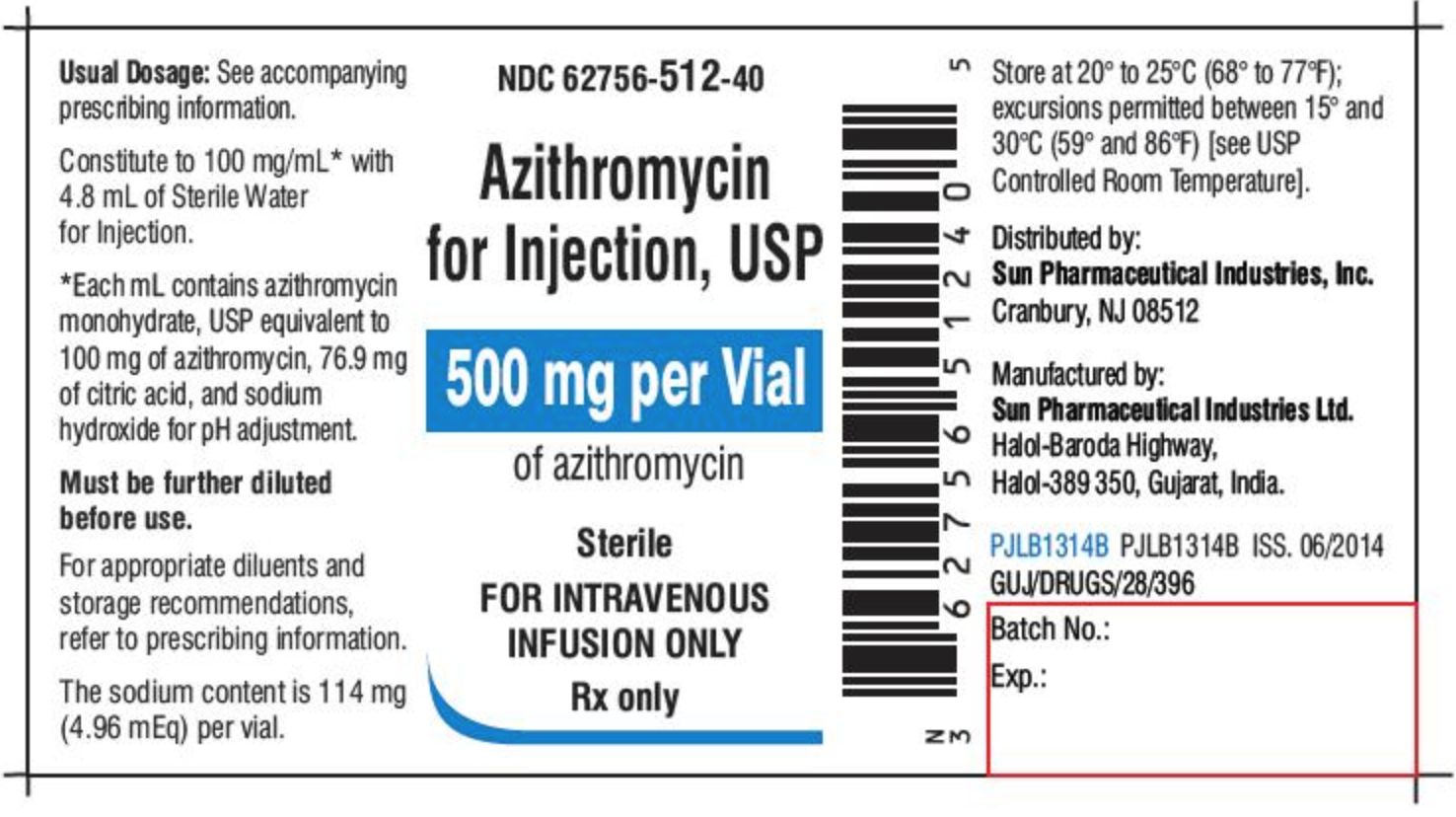
Medication labels must include
12 Must-Have OTC Drugs: Non-Prescription First Aid Supplies Acetaminophen is the most commonly recommended OTC medication for fever. It works well for minor aches and pains, especially for people who cannot tolerate anti-inflammatory medications such as ibuprofen or aspirin. It is important to read the labels in regard to the recommended dosing of each medication to prevent accidental overdose. FDA's Labeling Resources for Human Prescription Drugs | FDA Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective use of the drug; and (2) includes the Prescribing... How to Label Prescription Medication for Veterinary Patients A label should include the following components: The name of the veterinary practice, its address, and contact information The veterinarian's name, the patient's name and species, and the...
Medication labels must include. FDA Says Drug Labels Must Include Clear Guidance for ... - Healthline Starting June 30, new drug labels will have categories for "Pregnancy," "Lactation," and "Females and Males of Reproductive Potential." "Pregnancy" will include information on ... RXinsider | How to Label a Medication Syringe At a minimum, medication containers and medication syringe labels must include: • Name, address, and telephone number of the issuing pharmacy. Each piece of information is there to improve patient safety. Reducing spelling errors, typographical mistakes, or the spacing of the information including label breaks will help prevent patient harm. Drug labeling, Information about Drug labeling - FAQs Each product must contain a label with "Supplement Facts" in bold letters onthe front panel. This is the manufacturer's opportunity to identify the product. Below "Supplement Facts," the panel must state the serving size. This isdetermined by the manufacturer with no input from the FDA. Labeling guidelines for sample prescription drugs | Mass.gov Practitioners must label all sample medications dispensed to patients, including those provided as part of an indigent patient drug program (see M.G.L. c. 94C §22 and 105 CMR 700.010). Labels must contain the information described below; however, the method of labeling the medications may vary. For example, sample medications may be placed in ...
Labeling Information | Drug Products | FDA For more information on labeling, including Physician Labeling Rule (PLR) requirements, guidances, presentations, sample templates and format tools, and established pharmacologic class (EPC ... Forms in HTML documents - W3 17.1 Introduction to forms. An HTML form is a section of a document containing normal content, markup, special elements called controls (checkboxes, radio buttons, menus, etc.), and labels on those controls. Users generally "complete" a form by modifying its controls (entering text, selecting menu items, etc.), before submitting the form to an agent for processing (e.g., to a Web server, … What Information Should Be on Drug Labels? - MedicineNet Certain information must be included on a prescription drug label. The FDA requires prescription labeling to be printed with: Pharmacy information The doctor's information Instructions Physical description of the drug Federal caution statement Dates Pharmacy prescription number Number of pills Number of times the drug can be reordered How to Label a Medication Syringe - Medical Packaging Inc., LLC At a minimum, medication containers and medication syringe labels must include: Accurate spelling of medication name Brand name or generic name Patient's name Dosing amounts Dosing and/or drug administration instructions Total medication quantity Medication expiration date Date of dispensing Serial number Name of the prescriber
Safe Labeling Helps Prevent OR Medication Errors - OR Today The label should at a minimum include the drug's name and strength. 6 Some facilities require the scrub person's initials. When the drug is handed to the surgeon, the scrub person repeats the name and strength of the drug. The delivery device (syringe) is premarked by the manufacturer with dosage measurement increments for the surgeon's use. Best practice in the labelling and packaging of medicines In addition, it describes best practice in the area of labelling and packaging to ensure that medicines can be used safely by all patients, the public and healthcare professionals alike. It also ... What's on my medicine label? - Therapeutic Goods Administration (TGA) A doctor or pharmacist may include warnings for their patient on a prescription medicine by applying an additional label. Declarations. Some substances/ingredients found in medicines must be declared on the label. For example, potential allergens such as egg and fish must be declared if they are likely to be in the medicine. Cannabidiol (CBD): What we know and what we don't Sep 24, 2021 · Side effects of CBD include nausea, fatigue and irritability. CBD can increase the level of blood thinning and other medicines in your blood by competing for the liver enzymes that break down these drugs. Grapefruit has a similar effect with certain medicines. People taking high doses of CBD may show abnormalities in liver related blood tests.
Mixing Medications and Dietary Supplements Can Endanger Your … Jun 02, 2022 · Dietary supplements are widely used and include vitamins, minerals, and other less familiar substances—such as amino acids, botanicals, and botanical-derived ingredients.
Mental Health By the Numbers | NAMI: National Alliance on Mental … Mental health treatment—therapy, medication, self-care—have made recovery a reality for most people experiencing mental illness. Although taking the first steps can be confusing or difficult, it's important to start exploring options. ... Must travel 2x as far to their nearest hospital; Are 2x as likely to lack broadband internet, limiting ...
Medicines: packaging, labelling and patient information leaflets Labels must include warnings for safe use of the medicine. All products that contain paracetamol must include statutory warnings. Additional warning statements must be included on the...
Pharmaceutical Labeling 101: FDA Regulations Guide These include drugs like analgesics, anti-inflammatory agents, antibacterial, anticonvulsants, and others. The substance is used in the diagnosis, mitigation, cure, treatment, or prevention of diseases. This category also includes supplements. The substance is a component of medication but not a part of a medical device.
Forms in HTML documents - W3 17.1 Introduction to forms. An HTML form is a section of a document containing normal content, markup, special elements called controls (checkboxes, radio buttons, menus, etc.), and labels on those controls. Users generally "complete" a form by modifying its controls (entering text, selecting menu items, etc.), before submitting the form to an agent for processing (e.g., to a Web server, …
Water: How much should you drink every day? - Mayo Clinic Oct 12, 2022 · Your body loses fluids when you have a fever, vomiting or diarrhea. Drink more water or follow a doctor's recommendation to drink oral rehydration solutions. Other conditions that might require increased fluid intake include bladder infections and urinary tract stones. Pregnancy and breast-feeding.
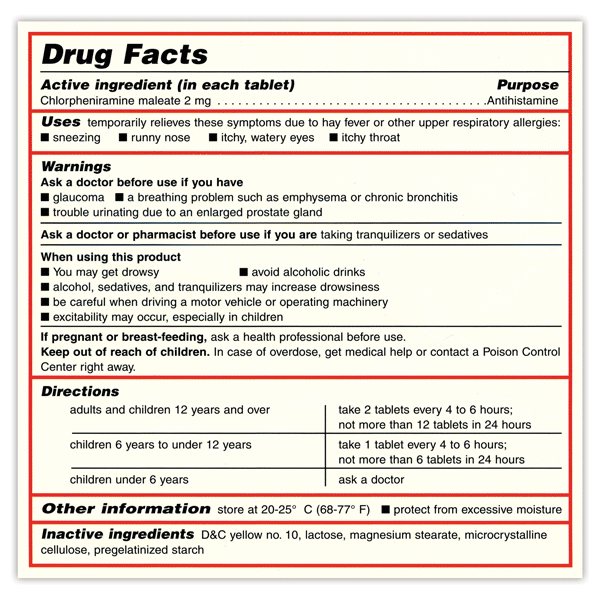
The Over-the-Counter Medicine Label: Take a Look | FDA All nonprescription, over-the-counter (OTC) medicine labels have detailed usage and warning information so consumers can properly choose and use the products. Below is an example of what the OTC...
Empty string - Wikipedia Formal theory. Formally, a string is a finite, ordered sequence of characters such as letters, digits or spaces. The empty string is the special case where the sequence has length zero, so there are no symbols in the string.
Chapter 5: Prescriptions and Labels Flashcards | Quizlet Drug Labels Regulated by the Food and Drug Administration (FDA), which determines what needs to be on the label Dispensing pharmacist's label must include: Pharmacy name, address, and phone number Dispensing date Dispensing date may differ from the date on the prescription. Rx number, which identifies this unique prescription in the computer system
Ch 8 Pharmacy Flashcards | Quizlet A legal prescription label must include all of the following except: A. Directions for use B. Date the prescription was dispensed C. Name, address, and telephone number of the prescriber D. Name, address, and telephone number of the dispensing pharmacy Name, address, and telephone number of the prescriber
Pharmaceutical Labeling Guide for FDA-Compliant Drug Labels The pharmaceutical labeling guidelines are found in Title 21 of the Code of Federal Regulations Part 201 (21 CFR 201), and the FDA strictly enforces them. Any misstep can render a drug misbranded. The regulations are different for prescription and over-the-counter (OTC) drugs. Below is an easy-to-understand walkthrough of the 21 CFR 201 guidelines.
Societal and cultural aspects of autism - Wikipedia The Aspie world, as it is sometimes called, contains people with Asperger syndrome (AS) and high functioning autism (HFA), and can be linked to three historical trends: the emergence of AS and HFA as labels, the emergence of the disability rights movement, and the rise of the Internet. Autistic communities exist both online and offline; many ...
How to Label Prescription Medication for Veterinary Patients A label should include the following components: The name of the veterinary practice, its address, and contact information The veterinarian's name, the patient's name and species, and the...
FDA's Labeling Resources for Human Prescription Drugs | FDA Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective use of the drug; and (2) includes the Prescribing...
12 Must-Have OTC Drugs: Non-Prescription First Aid Supplies Acetaminophen is the most commonly recommended OTC medication for fever. It works well for minor aches and pains, especially for people who cannot tolerate anti-inflammatory medications such as ibuprofen or aspirin. It is important to read the labels in regard to the recommended dosing of each medication to prevent accidental overdose.





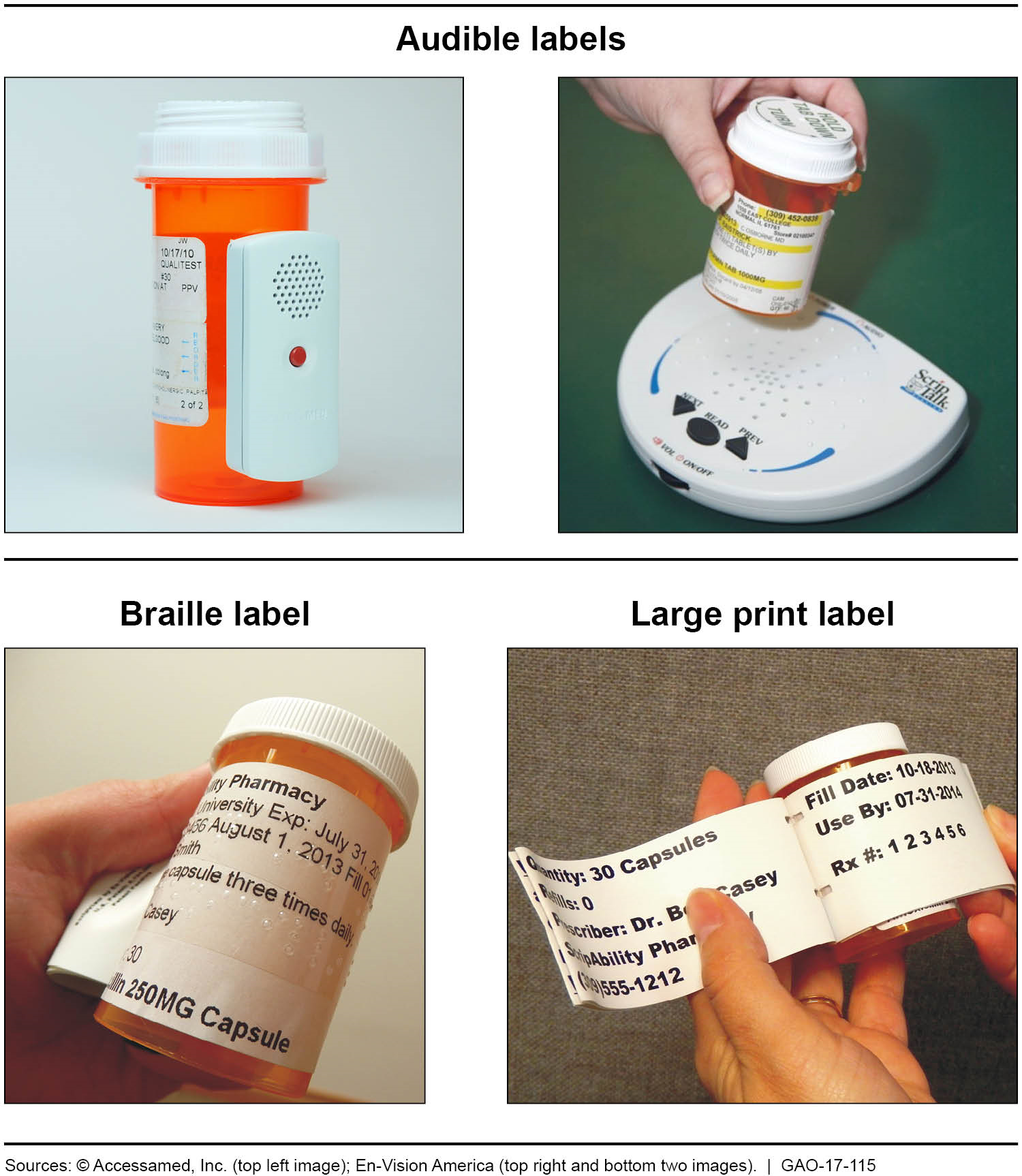

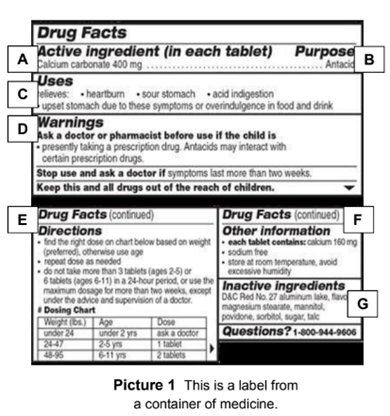





















Post a Comment for "39 medication labels must include"